Related Suggestion
Fapon Biotech Inc. (Fapon) announces the second COVID-19 donation to support the global pandemic battle with free Anti-SARS-CoV-2 Fully Human RBD IgM & IgG Quality Control Materials for the usage of 100 million tests. The first donation was initiated in quarter 1 to give 3 million PCR test components to solve the shortage of raw materials and test development challenges of global IVD partners.
The confirmed COVID-19 cases had exceeded 13 million with reagent manufacturers are ramping up antibody test production to massive scale per day. The antibody test is now not only applied to assess infection but also other medical fields. However, news of false negatives caused by antibody tests has never stopped.
In response to this problem, Fapon introduces HIGH SPECIFICITY and PURITY RBD IGM/IGG QC MATERIALS to meet the urgent demand for development and optimization of reliable quality controls for high-quality antibody tests. And to continue the company’s mission to Enable Earlier Disease Identification, More Convenient, Accurate and Affordable Diagnosis upon the fight of COVID-19, a new COVID-19 donation program is launched with the following details.
|
Eligible Applicant: |
SARS-CoV-2 antibody & neutralizing antibody test reagent manufacturer |
|
Product: |
Anti-SARS-CoV-2 fully human RBD IgM & IgG QC materials |
|
Application |
Reagent quality control and manufacture |
|
Quantity: |
1. QC material usage of 100 Million tests 2. Approx. 500–5000 K per eligible applicant after evaluation * Donated test volume is based on Fapon internal testing titer of the quality control on different platforms |
|
Application Process: |
1. Application submission via https://www.surveymonkey.com/r/FPQCDONATION 2. Fapon will review the application and contact the applicant for information verification 3. Quantity will be assigned to each eligible applicant after evaluation 4. Shipping arrangement |
|
Contact: |
market@fapon.com / +86-769-22898886-8105/8111/8110 |
* Fapon reserves the right of interpretation and change the arrangement of the donation
In The Heart of Reliable COVID-19 Antibody Tests——Fapon Anti-SARS-CoV-2 Fully Human RBD IgM & IgG QC Materials
Currently, the antibody test is playing essential roles in COVID-19 diagnosis and epidemiological study. The urgent need for vaccine and antibody-drug has also prompted the demand for neutralizing antibody test research and production. Fapon Anti-SARS-CoV-2 Fully Human RBD IgM & IgG QC Materials can provide performance references to both tests as a valuable tool to accelerate development, validation, and production. With most antibody detection kits in the market are developed based on the RBD binding domain, Fapon RBD IgM & IgG QC Materials can provide assessments to different antibody test devices and methods.
Product Features
-High batch-to-batch consistency, bulk volume per single batch
-Formulate based on the human matrix, analogous to patient samples
-High purity and activity
-Non-infectious, enabling safe handling
-Validated by different test platforms (CLIA/CMIA/ELISA/Lateral Flow)
Product Information
|
Catalog No. |
Description |
Recommended Method |
Platform |
|
FPZ0589 |
Anti-SARS-CoV-2 Fully Human RBD IgM Monoclonal Antibody |
Indirect/Capture/Sandwich |
CLIA/CMIA/ELSIA/ Lateral Flow |
|
FPZ0570 |
Anti-SARS-CoV-2 Fully Human RBD IgG Monoclonal Antibody |
Indirect/Capture/Sandwich |
CLIA/CMIA/ELSIA/ Lateral Flow |
Fapon IgM Monoclonal Antibodies (mAbs) CMIA Performance
1. The activity of Fapon IgM mAbs covered two COI levels within the concentration range 500 to 1000 ng/mL
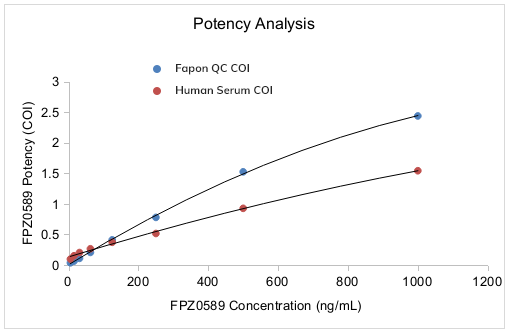
2. Fapon IgM mAbs demonstrated high purity and uniformity with no apparent difference in the precision between inner-bottle and bottle-to-bottle
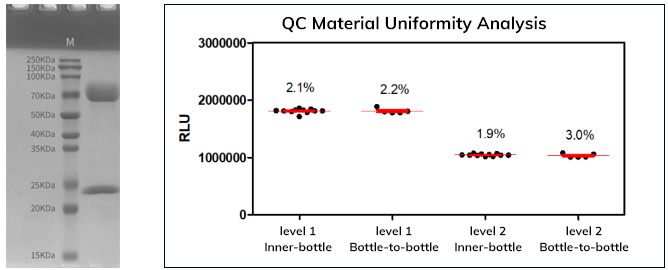
3.Fapon IgM mAbs exhibited excellent linearity within the dilution range 10 to 1000 ng/mL, suggested good ability to assess reagent performance variation
4. Fapon IgM mAbs demonstrated a consistent reaction pattern with the clinical human serum sample