Phase I (2001–2008)
Built the core technology platform of IVD raw materials, established the domestic leading position in the field of infectious disease diagnostic raw materials, and successfully entered global markets.
-
Phase I (2001–2008)
-
Phase II (2009–2016)
-
Phase III (2017–2020)
-
Phase Ⅳ (2021–Present)
-
Technology
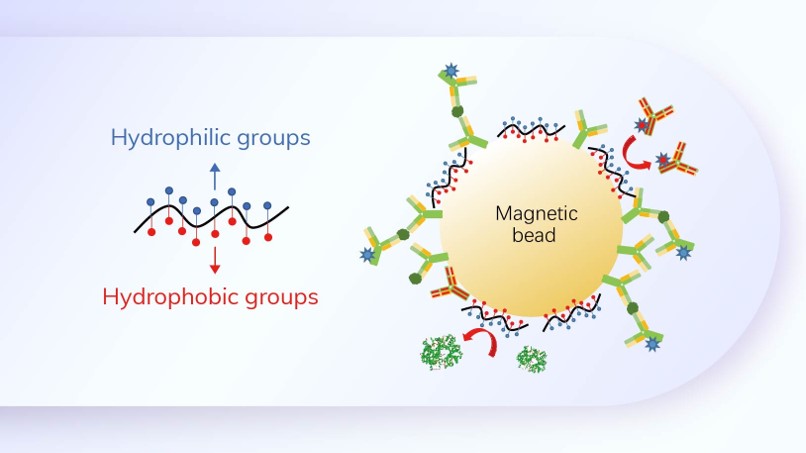
Realized recombinant protein expression by recombinant DNA and genetic engineering, built and perfected the prokaryotic and yeast expression platform;
Mastered the technology of the purification of prokaryotic and eukaryotic expressed protein, as well as the renaturation technology of protein, to develop antigen-based diagnostic raw materials;
Built platforms for monoclonal antibody development and colloidal gold detection, etc., and initiated exploration of one-stop solutions in 2007, laying the foundation for the raw materials diversification and the development of one-stop solutions.
-
Products
Diagnostic raw materials developed by Fapon Biotech are targeted for major infectious diseases such as HIV, HCV and TP were launched, covering the two traditional mainstream testing platforms of ELISA and colloidal gold. -
Innovation & Intellectual Property
Innovation ability was fully recognized in this phase when it was approved by the government as a high-tech enterprise in Shenzhen in 2005, and awarded the "Champion of the First Innovation Nanshan Entrepreneurs Star Contest" and the "Shenzhen Science and Technology Innovation Award" in 2008.
-
Market
Established the leading position in the field of
infectious disease diagnostic by marketing raw materials such as HIV, HCV, TP.
Also entered international markets in 2007.

Phase II (2009–2016)
The period of application platform expansion and underlying platform construction, providing clients with products and services of raw materials to one-stop solutions.
-
Phase I (2001–2008)
-
Phase II (2009–2016)
-
Phase III (2017–2020)
-
Phase Ⅳ (2021–Present)
-
Technology
During this phase, Fapon Biotech's
technological development was reflected in two main areas:
1. The establishment and improvement of
biotechnology platforms: the polyclonal antibodies and baculovirus-insect
expression systems, mammalian expression systems, genetically engineered
recombinant antibodies and other biotechnology platforms were established. A
variety of platforms for physical and chemical analysis, such as mass
spectrometry, high-performance liquid chromatography, ultra-performance liquid
chromatography and capillary electrophoresis, were also founded to guide the
development of R&D pipelines and assess the improvement of product
performance and quality control levels.
2. The construction of a series of testing
platforms: completed the construction of immunoturbidimetry, latex-enhanced
immunoturbidimetry, fluorescence-based qPCR, CLIA, sequencing and others, which
was equipped with the reagent raw material R&D capability of most testing
methodology in the field of IVD. While improving the raw material testing and
screening capabilities, the technology of the reagent solution was accumulated.
-
Products
Developed strong product lines of enzyme-linked
immunoassay, chemiluminescence, rapid test, molecular diagnostics,
biochemistry, etc., covering mainstream disease detection materials. Among
them, cTnI, malaria, CA 15-3 and many other products received high recognition
from customers for their excellent performance.
-
Innovation & Intellectual Property
Formally certified as a national high-tech
enterprise and won the "Guangdong Science and Technology Progress
Award" and the "Shenzhen Science and Technology Innovation
Award" in 2009. Another project was approved in 2010 and 2011 as the
"National Torch Plan" in 2010 and 2011. Another product was certified
as a key new product of Guangdong province.
-
Market
Maintained the position as the leading domestic
supplier of IVD raw materials while gradually expanding international markets.
Fapon Biotech became a mainstream raw material supplier to emerging markets and
entered markets of developed countries such as Europe and the US. Fapon Biotech
became a representative brand in the industry for the global export of Chinese
IVD raw materials.

Phase III (2017–2020)
Focuses on instrument and reagent platform development, and actively promotes diagnostic platform construction, gradually to form a comprehensive product line of one-stop solutions.
-
Phase I (2001–2008)
-
Phase II (2009–2016)
-
Phase III (2017–2020)
-
Phase Ⅳ (2021–Present)
-
Technology
Guided by the value of "Future
Anticipation," insights of future development trend, industry pain points,
and more, Fapon Biotech integrated cutting-edge technologies through the mode
of "co-creation and sharing" to provide clients with
high-performance, low-cost instrument and reagent solutions, building a highly
efficient diagnostic ecosystem. Since 2017, Fapon Biotech has focused on
building an instrument platform represented by fully automatic CLIA analyzers
and a series of reagent platforms for CLIA, biochemical diagnosis and molecular
diagnosis.
-
Products
During this phase, Fapon Biotech's product
development was reflected in two main areas:
1. Further diversification of raw materials:
Fapon Biotech continued to invest resources in raw material R&D projects
based on the demand of general health management and market trend, introducing
a series of new raw materials with continuous optimization, such as AMH, PG
I/II, HbA1c, cTnI, SAA, Malaria, influenza A, Dengue, NGS, etc.
2. Formation of a series of one-stop solutions:
the one-stop solutions of fully automated CLIA analyzer and reagent service, molecular
diagnosis and biochemical diagnosis were formally introduced to the market. The
business model of "Instrument + Reagent Service + Raw Material" with
personalized cooperation schemes created a new concept of one-stop solutions.
-
Innovation & Intellectual Property
In 2018, an HIV patented product won the
Chinese Patent Silver Award; in 2020, patented Taq Polymerase won the Guangdong
Province Patent Gold Award, and Fapon Biotech became a Shenzhen Patent
Advantage Enterprise and a Guangdong Province Intellectual Property
Demonstration Enterprise. Fapon Biotech has been paying great attention to
patent application and intellectual property protection. By the end of June
2020, Fapon Biotech applied for 377 patents with 80% for invention.
-
Market
Reached strategic cooperation with many
well-known domestic enterprises through the fully automatic CLIA analyzer, and
the instrument platform construction has achieved initial success. Meanwhile,
the advantages of one-stop solution that Fapon Biotech focused on creating were
fully reflected during the COVID-19 pandemic in 2020. Fapon Biotech beca,e the
mainstream supplier of COVID-19 PCR and antigen tests. Its raw materials and
semi-finished products supported a smooth supply of diagnostic reagents to the
end market, successfully helping over hundreds of IVD partners in the world
with their pandemic responses.

Phase Ⅳ (2021–Present)
Enhance IVD technologies and join hands with global partners to build a diagnostic ecosystem
-
Phase I (2001–2008)
-
Phase II (2009–2016)
-
Phase III (2017–2020)
-
Phase Ⅳ (2021–Present)
-
IVD Ecosystem
During this phase, Fapon Biotech is taking the
lead in building a diagnostic ecosystem. Based on the principle of not directly
involving into the terminal market, it integrates powerful bodies such as the
government, customs, universities, medical institutions, scientific research
institutes and so on to make every effort to place more open instruments for
IVD ecosystem partners. Fapon Biotech's core technical capabilities of core raw
materials, reagent solutions, and open instrument platforms, combined with
excellent ecosystem services and incubation funds, have provided fertile soil
for the growth of enterprises partner in the IVD ecosystem, jointly creating
open and inclusive industry environment for resource sharing.
-
Innovation & Intellectual Property
In 2021, "Fapon" has been included in
the Guangdong Province Key Trademark Protection List. During then, Fapon
Biotech paid more attention to patent application and intellectual property
protection. In 2022, its key technology of IVD reagents bioactive protein won
the “Second Prize of Guangdong Science and Technology Progress Awards.” As of
May 31, 2022, the total number of Fapon Biotech's patents reached 573, of which
inventions accounted for more than 80%.
-
Open Instrument Platforms and Product Breakthroughs
Fapon Biotech has deployed instrument platforms
on NGS and POCT molecular diagnostics, with the design concept to connect
upstream developers and downstream application users in an open operation mode,
accelerating technologies innovations and product iterations, so as to meet the
urgent needs of many IVD companies and terminal users. Sequlite, a wholly-owned
subsidiary of Fapon Biotech, has completed the research and development of the
first functional prototype of a desktop mid-to-low-throughput gene sequencing
instrument, and is currently testing and optimizing the beta model in the
commercialization stage. RunPon Bioscience, a subsidiary of Fapon Biotech, has
completed the registration inspection of the first mass-produced PCR analyzer
model and is pending approval of the registration certificate.
Fapon Biotech innovatively operates the
chemiluminescence analyzers in open mode. On one hand, it helps R&D and
production enterprises of chemiluminescence reagents build complete
chemiluminescence instrument and reagent systems more efficiently, and apply to
terminal medical testing institutions, accelerating their entrance to the
chemiluminescence market. On the other hand, Fapon Biotech also share the
instrument platform to the third-party reagent developers to promote the
development of adapting reagents and transformation to clinical applications in
a more efficient way. This will help promote industrial chain collaboration,
reduce instrument development costs, as well as improve industry operational
efficiency.
In addition, Fapon Biotech has launched complete
COVID-19 testing solutions which cover various testing application scenarios.
It provides IVD enterprises with one-stop COVID-19 detection technologies and
services ranging from reagent raw materials, semi-finished products to
technical support, registration assistance, etc., empowering local diagnostics on
an industrial level. Fapon Biotech closely tracks any emergence of new variant
and continuously improves the raw materials for antigen test. At present, its
antibody raw material for antigen test has iterated to its fifth-generation.
-
Market
With over 20 years of development, Fapon Biotech
has become an influential supplier of IVD reagent raw materials and solutions
worldwide. In recent years, Fapon Biotech's international business revenue
accounts for about 40%, winning trust from over 1,500 business partners around
the world, and its products are widely sold to 61 countries and regions in
Europe, America, Asia, Australia, Africa, etc.