Related Suggestion
Every year on April 25th, people around the world observe World Malaria Day, aimed at promoting global efforts in malaria prevention and control. However, despite concerted efforts over the past two decades, global malaria incidence remains alarmingly high.
According to the latest data from the World Health Organization, there were an estimated 249 million malaria cases worldwide in 2022, with numbers remaining unchanged from 2021. Particularly concerning is the heavy burden of malaria in the African region, which accounts for 94% of global malaria cases and 95% of malaria deaths.
Early diagnosis and treatment of malaria are crucial to reducing disease transmission and mortality. However, current malaria diagnostic methods face limitations, with one of the most prominent issues being false-negatives due to HRP2 gene deletions. HRP2 is a widely used antigen in rapid diagnostic tests (RDTs) for malaria, but increasing numbers of malaria parasites are showing HRP2 gene deletions, compromising test accuracy.
In addition to HRP2, lactate dehydrogenase (LDH) is another commonly used malaria parasite detection marker. While LDH sensitivity is typically lower than HRP2, its importance is heightened in the absence of HRP2. Therefore, the development of high-sensitivity LDH detection methods is imperative to ensure accurate malaria diagnosis.
In response to this challenge, the World Health Organization has put forward a series of recommendations, including transitioning to LDH-based testing and enhancing detection sensitivity. Furthermore, for malaria RDTs companies, the use of combined HRP2 and LDH detection methods is a worthy consideration.
Fapon's Commitment to Supporting Malaria Diagnostics
Since 2001, Fapon has been committed to researching core raw materials for infectious disease diagnostic reagents. Our malaria raw materials, benchmarked against leading international brands, deliver superior performance and have garnered favorable reviews from global partners.
For malaria-endemic regions without prevalent HRP2 gene deletion, we offer high-performance raw materials to facilitate WHO Prequalification (PQ) for mid-tier IVD reagent manufacturers. In regions with prevalent HRP2 gene deletion, we respond proactively to WHO's directives by developing highly sensitive Pf-PLDH raw materials that comply with WHO standards. Our product line includes various combinations of single and multiple lines to support manufacturers in obtaining WHO-PQ certification.
Pf-PLDH Raw Material Product List
|
Recommended Pairs |
Catalog No. |
Source |
Clonal No. |
Subtype |
Blocker |
Application |
|
|
Pair 1 |
BRCMALS216 |
CHO |
22D5 |
IgM |
HIER-R-040 |
Coating |
|
|
BRNMALN201 |
CHO |
25F2 |
IgM |
Conjugating |
|||
|
Pair 2 |
BRCMALS216 |
CHO |
22D5 |
IgM |
HIER-M-004 |
Coating |
|
|
BRCMALS212 |
Mouse |
6F10 |
IgG2b |
Conjugating |
|||
|
Pair 3 |
BRCMALS213 |
Mouse |
4C6 |
IgG2a |
HIER-M-004 |
Coating |
|
|
BRCMALS212 |
Mouse |
6F10 |
IgG2b |
Conjugating |
Pf-PLDH Product Performance
Features:
1. Pf-PLDH Single line: Internal control materials showed positive results LOD: 0.4 ng/mL. Samples from the NIBSC reference material 16/376 showed positive results LOD: 2IU/mL. Samples from the CDC panel showed positive results LOD: 20 parasites/μL.
2. Raw material products in the form of mixed-line testing are available.
3. Pf-PLDH, Pv-PLDH and Pf-HRPⅡ do not cross react with each other.
More details
|
|
Pf-pLDH Single line |
Pf-pLDH/HRPII Mixed line |
Pf/Pv Combo test |
|
|
Pf antibody for detection |
BRNMALN201 |
BRNMALN201+BRJMALS109 |
BRCMALS215+BRJMALS109 |
|
|
Pv antibody for detection |
/ |
/ |
BRNMALN202 |
|
|
Antibody for capture |
BRCMALS215 |
BRCMALS215+BRCMALS107 |
BRNMALN201+BRCMALS107 |
|
|
Buffer |
Sample buffer |
B |
B |
B/B |
|
NIBSC reference diluent buffer |
B |
B |
B/B |
|
|
Sensitivity (internal control material) |
Pf-LDH 10 ng/mL |
4 |
4 |
B/7 |
|
Pf-LDH 5 ng/mL |
5 |
5 |
B/8 |
|
|
Pf-LDH 2 ng/mL |
6 |
6 |
B/B |
|
|
Pf-LDH 0.4 ng/mL |
9 |
9 |
B/B |
|
|
Pf-HRP2 10 ng/mL |
B |
4 |
B/4 |
|
|
Pf-HRP2 2 ng/mL |
B |
7 |
B/7 |
|
|
Pf-HRP2 0.4 ng/mL |
B |
9 |
B/9 |
|
|
Pv-LDH 10 ng/mL |
B |
B |
6/B |
|
|
Pv-LDH 5 ng/mL |
B |
B |
7/B |
|
|
Pf Sensitivity test(NIBSC 16/376) |
PF 50 IU/mL |
4 |
4 |
B/7 |
|
PF 10 IU/mL |
6 |
6 |
B/9+ |
|
|
PF 5 IU/mL |
7 |
8 |
B/9 |
|
|
PF 2 IU/mL |
9 |
9 |
B/B |
|
|
PvSensitivity test(NIBSC 19/116) |
PV 50 IU/mL |
B |
B |
3/B |
|
PV 10 IU/mL |
B |
B |
5/B |
|
|
PV 5 IU/mL |
B |
B |
6/B |
|
|
PV 2 IU/mL |
B |
B |
7/B |
|
|
CDC panel (Parasites/μL) |
US 06F FC27/A3 2000 |
2+ |
1 |
B/1 |
|
US 06F FC27/A3 200 |
5 |
3 |
B/3- |
|
|
US 06F FC27/A3 20 |
9 |
7 |
B/8+ |
|
|
US 06F SANTA LUCIA 2000 |
3 |
2 |
B/2 |
|
|
US 06F SANTA LUCIA 200 |
7 |
4 |
B/4 |
|
|
US 06F SANTA LUCIA 20 |
9 |
8 |
B/9+ |
|
|
US 07F BENIN I 2000 |
2 |
1 |
B/2+ |
|
|
US 07F BENIN I 200 |
5 |
2 |
B/4 |
|
|
US 07F BENIN I 20 |
8 |
6+ |
B/7 |
|
|
US 07F PHI 2000 |
2 |
1 |
B/2+ |
|
|
US 07F PHI 200 |
5 |
3 |
B/3- |
|
|
US 07F PHI 20 |
B+ |
8 |
B/8- |
|
|
US 10F Nigeria XII 2000 |
2 |
1 |
B/2+ |
|
|
US 10F Nigeria XII 200 |
5 |
3 |
B/3- |
|
|
US 10F Nigeria XII 20 |
9 |
7 |
B/8 |
|
|
CDC mixed sample 200 |
4+ |
/ |
/ |
|
|
CDC mixed sample 20 |
8 |
/ |
/ |
|
|
Specificity |
n=200 |
0/200 |
1/200 |
0/200 |
*Patents have been applied for Fapon’s malaria raw material series.
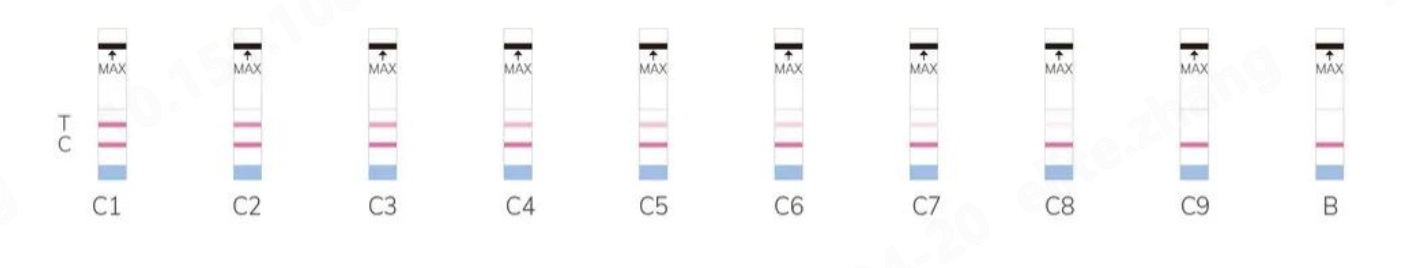
Colloidal Gold Standard Color Card
In addition to improving the sensitivity of Pf LDH, WHO also suggests improving the diagnosis through changes in product forms. Currently, the most recommended product form is the mix of Pf LDH+HRP2 and the combination of this mixed form and LDH-PV.
Fapon not only provides relevant raw material recommendations for traditional product combinations, but also introduces raw material solutions for new product forms, helping customers quickly complete new product development.
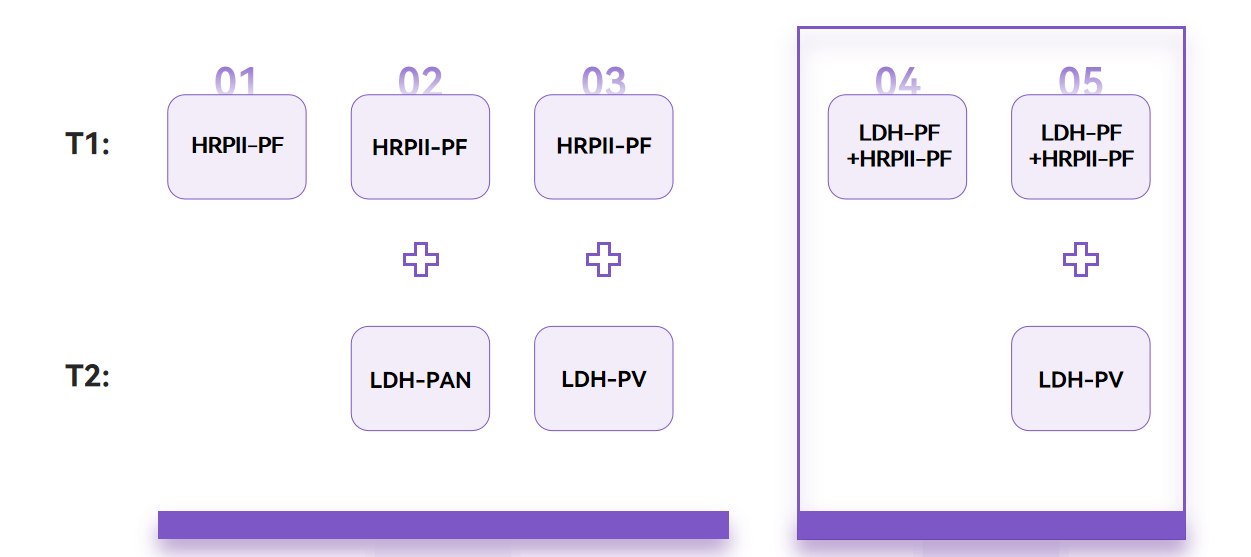
Traditional combinations (Left) and WHO new recommendations (Right)
Fapon offers options above
Fapon remains steadfast in its commitment to combat malaria by providing innovative and high-performance raw materials for diagnostic purposes. With our dedication to quality and adherence to global standards, we aim to play a significant role in the global effort to control and eventually eradicate malaria. Join us in the fight against this deadly disease, as together, we strive for a malaria-free world.
To learn more about our products, please visit our website http://en.faponbiotech.com:8081/ or contact us at market@fapon.com.
Appendix
Other raw material products for Malaria diagnosis
Project
Recommended Pairs
Catalog No.
Source
Clonal No.
Subtype
Blocker
Application
MA-HRPII-Pf
Pair 1
BRCMALS107
CHO
33C4
IgM
HIER-R-001
Coating
BRJMALS109
Mouse
18F7
IgG1
Conjugating
Pair 2
BRCMALS106
CHO
35A2
IgG1
HIER-R-001
Coating
BRJMALS109
Mouse
18F7
IgG
Conjugating
Pair 3
BRCMALS105
Mouse
7A11
IgM
HIER-R-001
Coating
BRJMALS106
Mouse
2B4
IgG1
Conjugating
Pair 4
BRCMALS105
Mouse
7A11
IgM
HIER-R-001
Coating
BRCMALS104
Mouse
7A3
IgG1
Conjugating
Pair 5
BRCMALS105
Mouse
7A11
IgM
HIER-R-001
Coating
BRJMALS107
Mouse
4A4
IgG1
Conjugating
Others
BRJMALS108
CHO
35H6
IgG1
/
Conjugating
MA-PLDH-PV
Pair 1
BRCMALS216
CHO
22D5
IgM
HIER-R-040 or HIER-R-039
Coating
BRNMALN202
CHO
25F8
IgM
Conjugating
Pair 2
BRCMALS216
CHO
22D5
IgM
HIER-M-004
Coating
BRCMALS211
Mouse
5B8
IgG1
Conjugating
Pair 3
BRCMALS213
Mouse
4C6
IgG2a
HIER-M-004
Coating
BRCMALS211
Mouse
5B8
IgG1
Conjugating
MA-PLDH-PAN
Pair 1
BRCMALS215
CHO
22D5
IgM
HIER-R040
Coating
BRJMALS214
CHO
37A8
IgG1
Conjugating
Pair 2
BRCMALS216
CHO
22D5
IgM
HIER-M-004
Coating
BRJMALS210
CHO
25C7
IgG1
Conjugating
Pair 3
BRCMALS216
CHO
22D5
IgM
HIER-M-004
Coating
BRJMALS212
CHO
--
--
Conjugating
Pair 4
BRCMALS216
CHO
22D5
IgM
HIER-M-004
Coating
BRJMALS204
Mouse
8A11
IgG1
Conjugating
Pair 5
BRCMALS213
Mouse
4C6
IgG2a
HIER-M-004
Coating
BRJMALS204
Mouse
8A11
IgG1
Conjugating
Pair 6
BRCMALS216
CHO
22D5
IgM
HIER-M-004
Coating
BRJMALS202
Mouse
1A5
IgG1
Conjugating
MA-PAN-PLDH+HRPII
Pair 1
BRCMALS216
CHO
22D5
IgM
HIER-M-004
Coating
BRCMALS107
CHO
33C4
IgM
Coating
BRJMALS210
CHO
25C7
IgG1
Conjugating
BRJMALS109
Mouse
18F7
IgG
Conjugating
Pair 2
BRCMALS213
Mouse
4C6
IgG2a
HIER-M-004
Coating
BRCMALS105
Mouse
7A11
IgM
Coating
BRJMALS204
Mouse
8A11
IgG1
Conjugating
BRJMALS106
Mouse
2B4
IgG1
Conjugating
Reference
1. Malaria. World Health Organization. Available at: https://www.who.int/zh/news-room/fact-sheets/detail/malaria (Accessed: 22 April 2024).
2. McCaffery, J.N. et al. (2021) Plasmodium falciparum PFHRP2 and PFHRP3 gene deletions among patients in the DRC enrolled from 2017 to 2018, Nature News. Available at: https://www.nature.com/articles/s41598-021-02452-3?error=cookies_not_supported&code=02e52307-d444-473f-8c7f-dfd03d02a609 (Accessed: 22 April 2024).
3. Sabin, S. et al. (2023) Portable and cost-effective genetic detection and characterization of Plasmodium falciparum HRP2 using the minion sequencer, Nature News. Available at: https://www.nature.com/articles/s41598-022-26935-z (Accessed: 22 April 2024).
4. Selecting and procuring malaria RDTS. Available at: https://www.who.int/teams/global-malaria-programme/case-management/diagnosis/rapid-diagnostic-tests/selection-and-procurement (Accessed: 22 April 2024).