Introduction
As a result of global efforts to combat the AIDS epidemic, significant progress has been achieved worldwide. According to UNAIDS, while 39 million people still live with HIV globally, the number of new infections has declined by 38% to 1.3 million in 2022 compared to 2010. Despite these improvements, the battle against HIV persists, as only 86% of newly infected individuals are aware of their HIV status, and approximately 630,000 people worldwide have died from AIDS-related illnesses. The significance of prevention, diagnosis and treatment in the ongoing fight remains paramount.
Detecting HIV subtypes for effective diagnosis
HIV, known for its genetic variability, comprises of two main types: HIV-1 that causes 95% of global infections, and HIV-2 with lower infectivity. This leads to diverse subtypes, numerous circulating recombinant forms (CRFs) and unique recombinant forms (URFs) globally, presenting challenges to the sensitivity and specificity of HIV testing. Therefore, developing diagnostic tests capable of detecting these variations is crucial for testing effectiveness.
Shortening window periods for punctual interventions
In addition to detecting various HIV strains, reducing diagnostic test window periods is crucial for timely treatment. The landscape of HIV testing has evolved significantly since the 1980s. Initially, individuals faced prolonged waiting periods, sometimes months, for detectable markers to appear. Advanced technologies now offer enhanced sensitivity, specificity, and shorter window periods through the latest generation test kits.
Notably, 4th generation HIV tests, detecting anti-HIV-1 and anti-HIV-2 antibodies along with the p24 antigen, are significantly advancing the detection window period to 2 to 3 weeks, enabling faster and more accurate diagnosis.
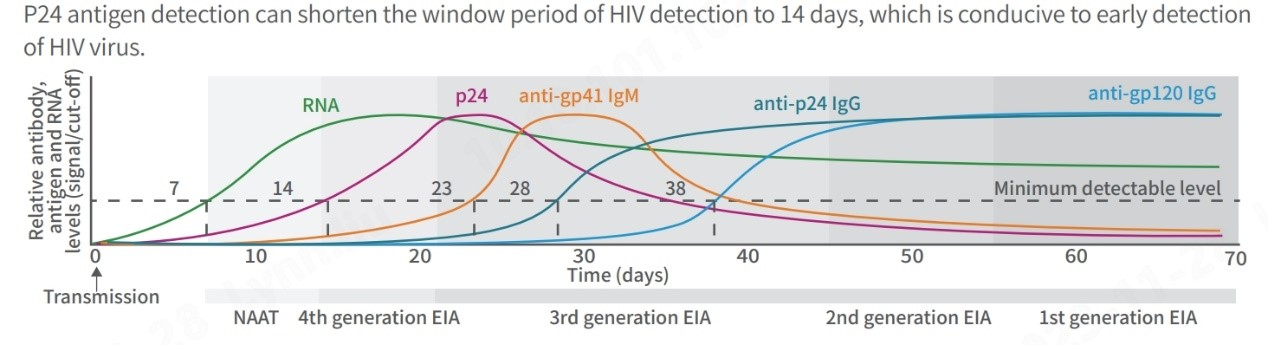
(Centers for Disease Control and Prevention, Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations Published June 27, 2014)
Moreover, 4th generation tests that detect the p24 antigen is recommended for diverse scenarios, such as early detection for infants under 18 months of age, shortening the window period for adults in acute and high-risk phases and individuals using self-test kits, and evaluating responses in those participating in HIV vaccine trials.
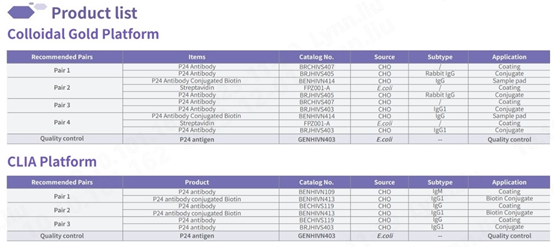
Raw materials for detecting p24
Our IVD raw materials for detecting the p24 antigen exhibit outstanding sensitivity and specificity, comparable to global renowned brands. Capable of detecting major HIV strains circulating worldwide, including common subtypes, our products are tailored to meet specific requirements.
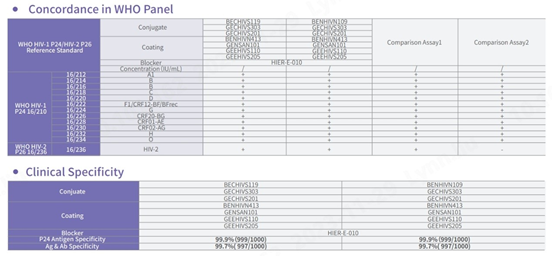
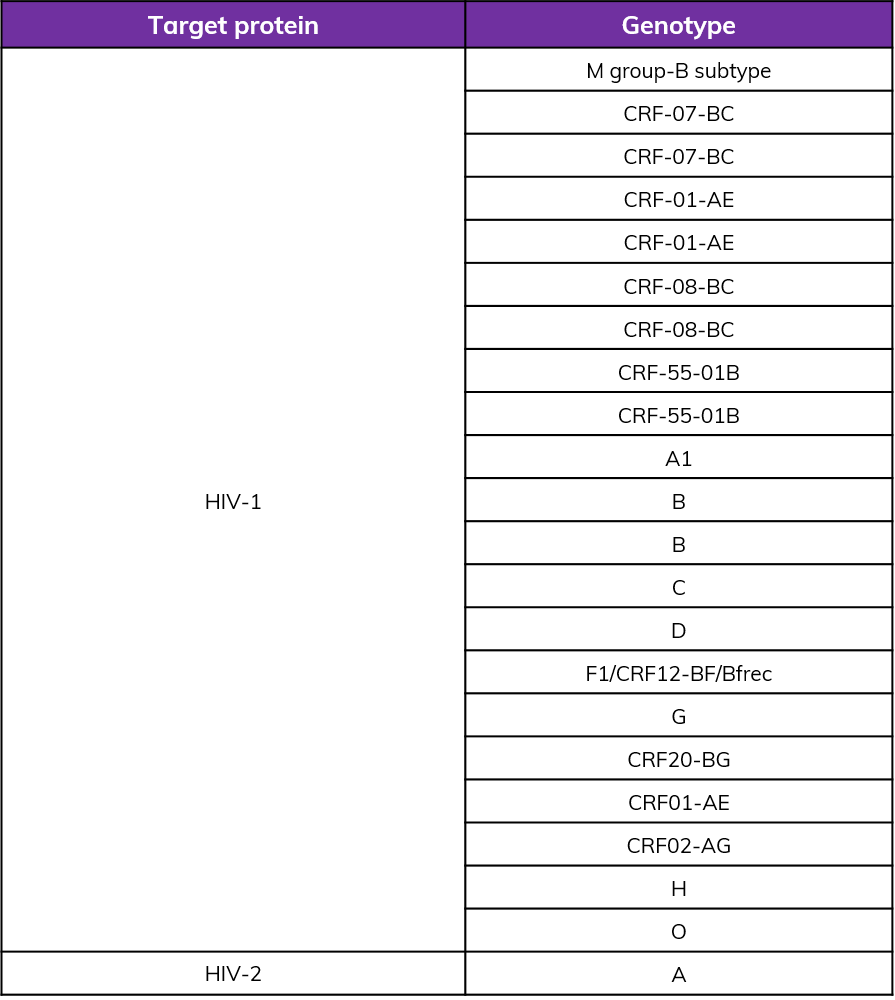
HIV subtypes detected

Performance
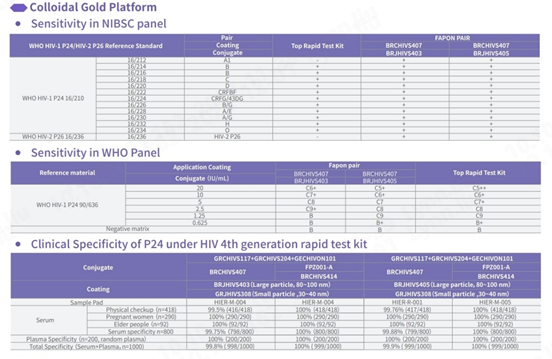
Sensitivity and specificity tested with NIBSC reference materials
In comparison with HIV test kits from other world-leading brands, our product demonstrates proficiency in detecting all subtypes of HIV-1 and HIV-2, especially in the NIBSC reference materials coded 16/212 and 16/236, ensuring comprehensive detection. In addition, the specificity of this product demonstrates outstanding performance, with an overall specificity exceeding 99.8%.

As we pioneer advancements in HIV detection, we are dedicated to delivering earlier, more accurate, convenient and accessible diagnostics for all. For further details on our innovative and diverse product range, please contact our sales representatives. We look forward to discussing how our solutions can make a difference for you!
Appendix
References
1. About HIV testing. Available at: https://www.21171069.gov.hk/en/facts_figures/testing.html (Accessed: 29 November 2023).
2. Akahome, P. (2021) HIV-1 subtypes, aidsmap.com. Available at: https://www.aidsmap.com/about-hiv/hiv-1-subtypes (Accessed: 29 November 2023).
3. Antiretroviral therapy for HIV infection in infants and children: Towards universal access: Recommendations for a public health approach (no date) National Center for Biotechnology Information. Available at: https://pubmed.ncbi.nlm.nih.gov/23741772/ (Accessed: 29 November 2023).
4. Global HIV statistics - joint united nations programme on HIV/AIDS. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (Accessed: 29 November 2023).
5. Laboratory testing for the diagnosis of HIV infection : Updated recommendations, Centers for Disease Control and Prevention. Available at: https://stacks.cdc.gov/view/cdc/23447#:~:text=The%20recommended%20algorithm%20is%20a,and%20HIV%2D1%20p24%20antigen. (Accessed: 29 November 2023).
6. Robertson, D.L., Hahn, B.H. and Sharp, P.M. (no date a) Recombination in AIDS viruses - journal of molecular evolution, SpringerLink. Available at: https://link.springer.com/article/10.1007/BF00163230 (Accessed: 29 November 2023).
7. The evolution of HIV testing (no date a) ADLM (formerly AACC). Available at: https://www.myadlm.org/cln/articles/2017/september/the-evolution-of-hiv-testing (Accessed: 29 November 2023).
8. VISR Working Group of Global HIV Vaccine Enterprise et al. (2015) HIV vaccine-induced sero-reactivity: A challenge for trial participants, researchers, and physicians, Vaccine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4331207/ (Accessed: 29 November 2023).
Join us for a better future of IVD.
If you have any question or need any support, please fill out the information below.