Related Suggestion
By early winter in 2022, RSV, flu, and COVID-19 were surging more or less simultaneously. It is likely that this is going to be the new normal with each year seeing the co-circulation and seasonality of RSV, flu, and COVID-19. Identifying which virus is causing an infection is critical to appropriate medical management and necessary for properly tracking outbreaks.
Trend: FDA
Recommends At-home COVID-19 Serial Rapid Test
February 24, 2023, the
U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA)
for the first over-the-counter (OTC) at-home diagnostic test that can
differentiate and detect influenza A and B, commonly known as the flu, and
SARS-CoV-2, the virus that causes COVID-19.
The authorization of the first OTC test that can detect Influenza A and B, along with SARS-CoV-2, is a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home. The FDA strongly supports innovation in test development, and is eager to continue advancing greater access to at-home infectious disease testing to best support public health needs.
The collective impact of COVID-19, flu and RSV
underscore the importance of diagnostic tests for respiratory viruses, and the
FDA recognizes the benefits that home testing can provide. The agency will
continue to use its authorities to increase the number of appropriately
accurate and easy-to-use at-home tests available to the public, especially
tests that detect these highly contagious respiratory viruses.
What Can
Fapon Help?
Fapon Biotech Inc. has been working on the core reagent raw materials for over 20 years, constantly innovating and iterating on the technology and products. Fapon Biotech has spared no effort in the research and development of raw materials for respiratory diseases, especially for SARS-CoV-2, influenza and RSV. We also provide different raw materials choices for SARS-CoV-2 & FLU A & FLU B combo in the rapid test.
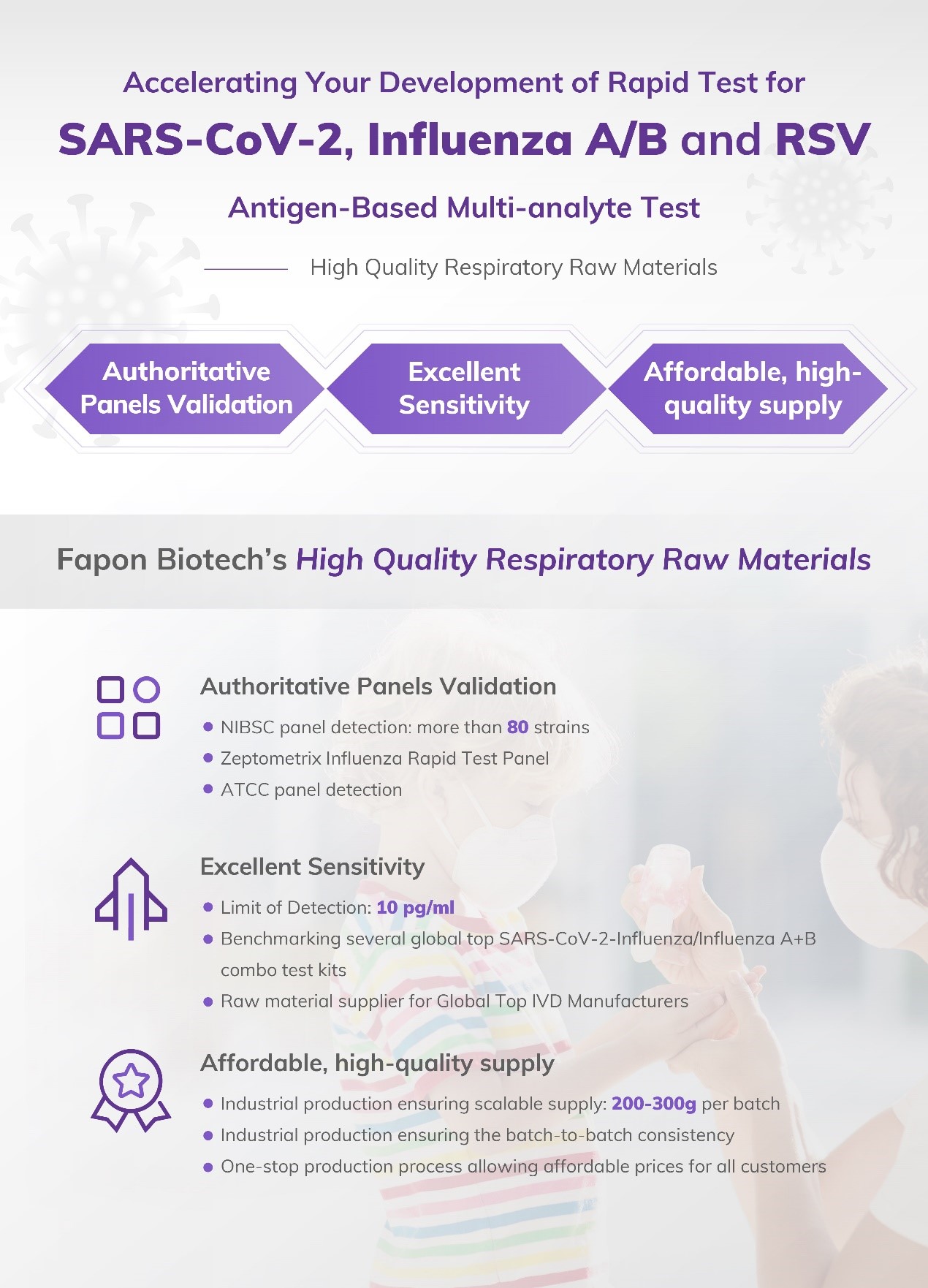
Fapon Biotech's Quality Raw Materials for Respiratory Detection
SARS-CoV-2
Item
Catalog No.
Source
Clonal No.
Subtype
Blocker
Application
Fifth generation product
FPZ0641
\
\
\
HIER-R-015/HIER-R-016
Coating
FPZ0834
CHO
28A1
IgG(Modified)
Conjugate
FPZ0861
\
\
\
Pad treatment
Fourth generation product
FPZ0831
CHO
35B7
IgM
Coating
FPZ0834
CHO
28A1
IgG(Modified)
Conjugate
Third generation
FPZ0812
Rabbit
35F12
IgG
Coating
FPZ0811
Mouse
14A7
IgG1
Conjugate
Second generation
FPZ0639
CHO
31F11
IgM
Coating
FPZ0638
CHO
31F12
IgM
Conjugate
First generation
FPZ0546
Mouse
5E7
IgG1
Coating
FPZ0574
Mouse
8F5
IgG1
Conjugate
First generation
FPZ0546
Mouse
5E7
IgG1
Coating
FPZ0573
Mouse
8A8
IgG1
Conjugate
Influenza A/B
|
Item |
Pair No. |
Catalog No. |
Source |
Clonal No. |
Subtype |
Blocker |
Application |
Platform |
|
|
Colloidal Gold |
Immunofluorescence |
||||||||
|
FLUA |
Pair 1 |
BRCINFS106 |
CHO |
33C8 |
IgG2b |
√ |
Coating |
√ |
√ |
|
BRJINFS104 |
CHO |
27A5 |
IgG2a |
√ |
Conjugate |
√ |
√ |
||
|
Pair 2 |
BRCINFS104 |
CHO |
35C1 |
IgM |
√ |
Coating |
√ |
√ |
|
|
BRCINFS105 |
CHO |
34D3 |
IgG1 |
√ |
Conjugate |
√ |
√ |
||
|
Pair 3 |
BRJINFS103 |
CHO |
36G1 |
IgM |
√ |
Coating |
√ |
√ |
|
|
BRCINFS105 |
CHO |
34D3 |
IgG1 |
√ |
Conjugate |
√ |
√ |
||
|
Pair 4 |
BRCINFS102 |
CHO |
31B4 |
IgG1 |
√ |
Coating |
√ |
√ |
|
|
BRCINFS104 |
CHO |
35C1 |
IgM |
√ |
Conjugate |
√ |
√ |
||
|
Pair 5 |
BRCINFS102 |
CHO |
31B4 |
IgG1 |
√ |
Coating |
√ |
√ |
|
|
BRCINFS103 |
CHO |
30A5 |
IgG2a |
√ |
Conjugate |
√ |
√ |
||
|
Pair 6 |
BRJINFS102 |
CHO |
30C6 |
IgG2a |
√ |
Coating |
√ |
√ |
|
|
BRCINFS102 |
CHO |
31B4 |
IgG1 |
√ |
Conjugate |
√ |
√ |
||
|
Pair 7 |
BRCINFS102 |
CHO |
31B4 |
IgG1 |
√ |
Coating |
√ |
√ |
|
|
BRJINFS102 |
CHO |
30C6 |
IgG2a |
√ |
Conjugate |
√ |
√ |
||
|
FLUB |
Pair 1 |
BRNINFC205 |
CHO |
22A9 |
IgM |
√ |
Coating |
√ |
√ |
|
BRNINFJ208 |
CHO |
25C4 |
IgM |
√ |
Conjugate |
√ |
√ |
||
|
Pair 2 |
BRNINFC204 |
CHO |
25D1 |
IgG1 |
√ |
Coating |
√ |
√ |
|
|
BRNINFJ207 |
CHO |
21F3 |
IgG2a |
√ |
Conjugate |
√ |
√ |
||
|
Pair 3 |
BRNINFC201 |
Mouse |
2F3 |
IgM |
√ |
Coating |
√ |
√ |
|
|
BRNINFJ203 |
CHO |
30D4 |
IgG2a |
√ |
Conjugate |
√ |
√ |
||
|
Pair 4 |
BRNINFC201 |
Mouse |
2F3 |
IgM |
√ |
Conjugate |
√ |
√ |
|
|
BRNINFJ203 |
CHO |
30D4 |
IgG2a |
√ |
Coating |
√ |
√ |
||
|
Pair 5 |
BRNINFC201 |
Mouse |
2F3 |
IgM |
√ |
Coating |
√ |
√ |
|
|
BRNINFJ204 |
CHO |
30E2 |
IgG2b |
√ |
Conjugate |
√ |
√ |
||
Influenza A+B performance
|
Pair |
Pair 1 |
Pair 3 |
Pair 4 |
Pair 5 |
||||||
|
Type |
NIBSC Strains |
Conjugating |
BRCINFS 104 |
BRNINFJ 208 |
BRCINFS 104 |
BRNINFC 201 |
BRCINFS 104 |
BRNINFJ 203 |
BRCINFS 104 |
BRNINFC 201 |
|
Coating |
BRCINFS 106 |
BRNINFC 205 |
BRCINFS 102 |
BRNINFJ 203 |
BRCINFS 102 |
BRNINFC 201 |
BRCINFS 102 |
BRNINFJ 204 |
||
|
A |
H1N1 |
Influenza Virus Infectious CNIC-1909 (H1N1) |
2+ |
3+ |
3 |
3+ |
||||
|
X-349 reassortant derived from A/Indiana/02/2020 H1N1 |
4 |
5+ |
5 |
5+ |
||||||
|
Influenza Antigen A/New Caledonia/20/99 H1N1 |
6 |
7 |
8 |
7 |
||||||
|
IVR-217 reassortant derived from A/Victoria/1/2020 H1N1 |
6+ |
7+ |
7 |
7+ |
||||||
|
A/Victoria/2570/2019 H1N1 |
6+ |
7+ |
7 |
7+ |
||||||
|
Influenza Virus Infectious A/California/7/09 44430 E6 H1N1 |
4 |
5+ |
5 |
5+ |
||||||
|
H2N2 |
A/Singapore/1/1957 H2N2 |
4 |
5 |
6 |
5 |
|||||
|
H3N1 |
A/mallard/England/727/2006 H3N1 |
2 |
3 |
4+ |
3 |
|||||
|
H3N2 |
Influenza Virus Infectious X-359 (H3N2) |
2+ |
3+ |
3 |
3+ |
|||||
|
Influenza Antigen A/Victoria/361/2011 (H3N2)(IVR-165) |
5 |
6 |
7 |
6 |
||||||
|
IVR-216
reassortant derived from A/Victoria/3/2020 (A/Victoria/3/2020) (H1N1) x |
7+ |
7+ |
7 |
7+ |
||||||
|
Influenza virus infectious A/SouthAustralia/34/2019 H3N2 |
6 |
6 |
7 |
6 |
||||||
|
A/Cambodia/E0826360/2020 H3N2 |
4 |
4 |
5 |
4 |
||||||
|
A/Tasmania/503/2020 H3N2 |
3 |
3 |
4 |
3 |
||||||
|
A/Darwin/6/2021 H3N2 |
4 |
4 |
5 |
4 |
||||||
|
Influenza Virus Infectious A/Hong Kong/2671/2019 H3N2 |
4 |
4 |
5 |
4 |
||||||
|
IVR-228 H3N2 |
6 |
7+ |
7 |
7+ |
||||||
|
A/Darwin/9/2021 H3N2 |
2 |
3+ |
3 |
3+ |
||||||
|
H5N1, H1N1 |
A/Hong Kong/213/2003 (H5N1) and A/PR/8/34(H1N1) |
2 |
4+ |
5+ |
4+ |
|||||
|
H5N1 |
A/Vietnam/1194/2004 H5N1 |
4 |
5+ |
5 |
5+ |
|||||
|
A/turkey/Turkey/1/2005 H5N1 |
4+ |
5+ |
5 |
5+ |
||||||
|
A/Cambodia/R0405050/2007 H5N1 |
2+ |
3+ |
3 |
3+ |
||||||
|
A/Anhui/1/2005 H5N1 |
1 |
2 |
3 |
2 |
||||||
|
H5N3 |
A/Duck/Singapore-Q/F119-3/97(H5N3) |
4 |
6+ |
6 |
6+ |
|||||
|
H7N1 |
A/turkey/Italy/3889/1999 H7N1 |
4 |
4 |
5+ |
4 |
|||||
|
A/mallard/Netherlands/12/2000 H7N1 |
5 |
6 |
7 |
6 |
||||||
|
H7N2 |
A/New York/107/2003 H7N2 |
2 |
3+ |
3 |
3+ |
|||||
|
H7N3 |
A/mallard/Netherlands/12/2000 H7N3 |
4 |
5+ |
5 |
5+ |
|||||
|
H7N9 |
A/Shanghai/2/2013 H7N9 |
4+ |
5+ |
5 |
5+ |
|||||
|
A/Anhui/1/2013 H7N9 |
2 |
3 |
4 |
3 |
||||||
|
H9N2 |
A/HK/1073/99 (G1 lineage) H9N2 |
4+ |
5++ |
5 |
5++ |
|||||
|
A/chicken/Hong Kong/G9/1997 (G9 lineage)H9N2 |
2+ |
3+ |
3 |
3+ |
||||||
|
B |
Influenza Virus infectious B/Florida/4/2006 BV |
5 |
7 |
7+ |
7 |
|||||
|
Influenza virus infectious B/Malaysia/2506/2004 BV |
2 |
4+ |
3 |
4+ |
||||||
|
B/Washington/02/2019 Wild-type virus BV |
4 |
7+ |
6 |
7+ |
||||||
|
B/Brisbane/35/2018 BV |
6 |
8 |
8+ |
8 |
||||||
|
CNIC-1906 derived from B/Sichuan-Gaoxin/531/2018 BV |
6 |
8 |
8+ |
8 |
||||||
|
B/Victoria/705/2018 Wild-type virus BV |
9+ |
B |
B |
B |
||||||
|
Influenza Virus Infectious BVR-25 (B-Victoria Lineage) BV |
9 |
B |
B |
B |
||||||
|
B/Singapore/WUH4618/2021 BV |
4+ |
6+ |
5 |
6+ |
||||||
|
B/Phuket/3073/2013 wild type virus BV |
4 |
6 |
6+ |
6+ |
||||||
|
B/Hong Kong/574/2019 Wild-type virus BV |
7+ |
9 |
9+ |
9 |
||||||
|
Influenza Virus Infectious BX-97A (B-Victoria Lineage) B/Slovenia/1584/2020 BV |
7 |
9 |
9+ |
9 |
||||||
|
Influenza Virus Infectious BX-93B (B-Victoria Lineage)BV |
6 |
8+ |
8 |
7 |
||||||
|
B/Utah/9/2014 wild type virus BY |
7+ |
9+ |
8 |
9+ |
||||||
|
NYMC BX-59A reassortant derived from B/California/12/2015 BY |
7+ |
9+ |
8 |
9+ |
||||||
|
NYMC BX-63 reassortant derived from B/Arizona/10/2015 BY |
7+ |
9 |
9+ |
9 |
||||||
|
B/Singapore/INFTT-16-0610/2016 (cell derived) antigen BY |
6 |
8+ |
8+ |
8+ |
||||||
|
B/Brisbane/9/2014 wild type virus BY |
4 |
5 |
5 |
6+ |
||||||
|
NYMC BX-57 reassortant derived from B/Hong Kong/3417/2014 BY |
4+ |
6+ |
5+ |
6+ |
||||||
|
B/Michigan/01/2021 BY |
5 |
6 |
6 |
7+ |
||||||
|
B/Darwin/7/2019 (cell derived) antigen BV |
7+ |
9 |
8 |
9 |
||||||
Note. The data number means the color developed by each pair. The color gets lighter, the number gets higher, which representing that the corresponding sensitivity is lower. And vice versa.
Respiratory syncytial virus
|
Item |
Pair No. |
Catalog No. |
Description |
Source |
Clonal No. |
Subtype |
Application |
Platform |
|
|
Colloidal Gold |
ELISA |
||||||||
|
RSV |
Pair 1 |
BRNRSVN103 |
Anti-Respiratory Syncytial virus F protein antibody |
CHO |
36F8 |
IgG2b |
Coating |
√ |
- |
|
BRNRSVN104 |
Anti-Respiratory Syncytial virus F protein antibody |
CHO |
33D12 |
IgG2a |
Conjugate |
||||
|
Pair 2 |
BRNRSVN102 |
Anti-Respiratory Syncytial virus N protein antibody |
CHO |
35B9 |
IgM |
Coating |
√ |
- |
|
|
BRNRSVN101 |
Anti-Respiratory Syncytial virus N protein antibody |
CHO |
30A2 |
IgM |
Conjugate |
||||
|
Pair 3 |
FPZ0181 |
Anti-Respiratory Syncytial virus N protein antibody |
Mouse |
-- |
IgG2b |
Coating |
√ |
√ |
|
|
FPZ0180 |
Anti-Respiratory Syncytial virus N protein antibody |
Mouse |
-- |
IgG2a |
Conjugate |
||||
|
Supporting Antigen |
HRSV |
FPZ0645 |
Respiratory Syncytial virus antigen |
- |
- |
- |
Quality Control |
|
|
To know more details about Fapon Biotech's Quality Raw Materials for Respiratory Detection, please contact us!
Email: market@fapon.com
Tel: +86 769 22898886