The Urgent Need to Address the Threat of High Prevalence (≥5%) of Malaria Pfhrp2/3 Gene Deletions
April 25, 2023 marks the 16th World Malaria Day. According to the World Malaria Report 2022[1] released by WHO, the total number of global malaria cases amounted to 247 million in 2021, with about 619,000 deaths; and the total number of global malaria cases amounted to 245 million in 2020, with approximately 625,000 deaths. The WHO African region still accounts for a high proportion of the global malaria burden. In 2021, African region accounted for 95 percent of the total malaria cases and 96 percent of the total malaria deaths, in which children under five years old accounted for approximately 80 percent of the total malaria deaths in the region.
There are five species of parasites that cause human malaria, among which Plasmodium falciparum (P.falciparum, PF) and Plasmodium vivax (P.vivax, PV) pose the greatest threat to human health. Early diagnosis and treatment of malaria can reduce transmission and deaths. Microscopy and rapid diagnostic tests (RDTs) are commonly used for malaria diagnosis. RDTs has become the mainstream testing method because of its operational convenience and low cost. The main targets of RDTs for malaria testing are Histidine Rich Protein II (HRP-II, specific to PF) and malaria parasite lactate dehydrogenase (LDH). RDTs for HRP-II antigen testing is dominant. Single-line tests reliant on this one antigen represented 80% of the 413 million RDTs procured from WHO-PQ suppliers in 2021[2].
However, there are still two pain points surrounding malaria testing using RDTs:
1. The spread of P.falciparum malaria with Pfhrp2/3 gene deletion poses a major threat to originally reliable diagnostics, and WHO recommends a shift to non-HRP2 tests in regions where 5% or more of P.falciparum are false negative on HRP2 RDTs due to Pfhrp2/3 gene deletions. In addition, it is not possible to identify treatment success in a timely manner as PF-HRP2 persists in the blood for 1-5 weeks after effective treatment. To confirm treatment results, it is necessary to transfer the patients to the organization with microscopy or PF-LDH-based RDTs;
2. Currently, available RDTs are not sensitive enough to detect LDH targets on P.falciparum and P.vivax. Therefore, WHO has focused on improving the sensitivity of species-specific LDH biomarkers in order to meet the urgent need for non-HRP2 tests of P.falciparum and P.vivax[3].
HRP2 gene deletions have accounted for more than 5% in some countries and regions. Fapon Biotech has been paying close attention to the worldwide malaria situation and actively responding to the call of WHO. In regards to the above pain points, Fapon Biotech is committed to developing highly sensitive raw materials for LDH tests, and continuously upgrading HRP2 pairs and maintaining world-leading product pairs, and will constantly upgrade and optimize the products to contribute to the eradication of malaria.

1. Devotion to addressing the threat of false negative rate (≥5%) of malaria testing due to Pfhrp2/3 gene deletions
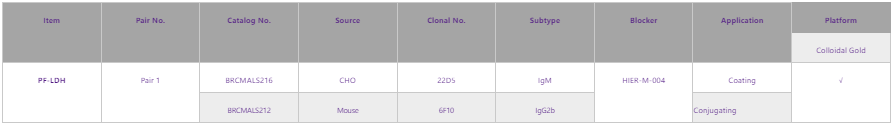
The lower limit of new competitive tests for P.vivax and P.falciparum LDH is 5-10 times that of previous tests and should be less than 1 ng/mL[4]. Therefore, in order to solve the problem of false negative rate caused by HRP2 gene deletions, Fapon Biotech will develop products for testing 1 ng/mL PF-LDH antigen, and devote itself to addressing the problem of false negative rate of P.falciparum caused by Pfhrp2/3 gene deletions. The pairs and performance data of PF-LDH featured products from Fapon Biotech are shown below:
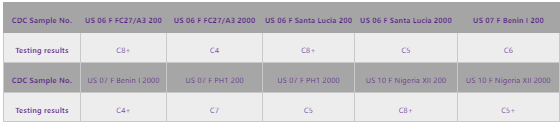
Samples from WHO-CDC disks were tested using this pair, showing activity in the data below:
Specificity: 200 negative samples were tested using this pair, showing no false positive result;
Corss reaction: PV positive samples and HRP2 positive samples were tested using this pair, showing no cross reaction;
2. Maintaining the leading level of raw materials
PF-HRP-II has high sensitivity and high specificity of P. falciparum malaria. Compared with other detection markers, HRP-II detection in regions without HRP2 gene deletion is a more recommended means of detection. Fapon Biotech has continuously upgraded the performance of product pairs for PF-HRP2, and it has retained a world-leading level. At present, the main product pairs of Fapon Biotech are shown below:
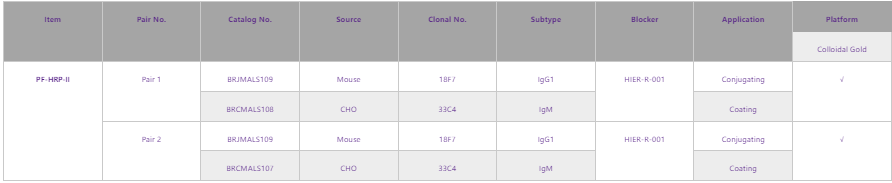
Download promotional flyers for HRP2:https://drive.google.com/file/d/1teck_pVVc94cQPCSTZMmEaS3mxLxhKOV/view?usp=sharing
3. Continuous iteration and upgrade
In addition to the above testing raw materials for PF, Fapon Biotech also supplies those for PV and PAN. The main product pairs are shown below:
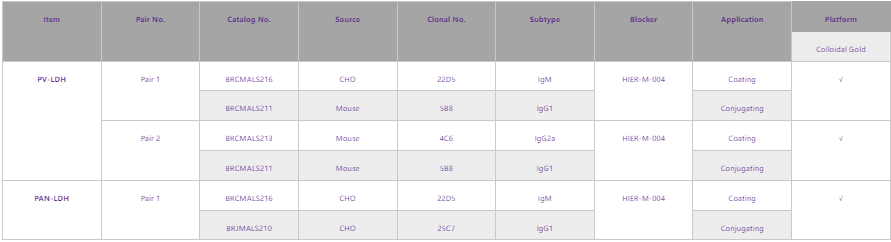
Fapon Biotech will continuously keep an eye on the changes of market dynamics, constantly upgrade and optimize products to ensure effective raw materials and timely supply, and maintain sharp market acumen. Meanwhile, Fapon Biotech welcomes more partners to work with us in the contribution to eradicating malaria.
[1] [2] [3] Information Source:World Malaria Report 2022:https://www.who.int/publications/i/item/9789240064898
[4] Information Source: Malaria RDT workshop for Chinese manufacturers---PATH2022