Related Suggestion
Fapon Biotech makes persist efforts to deal with the global pandemic new challenge, introduces COVID-19 Variant E484K POCT Solution for the effective differentiation of E484K and non-E484K infection. Previously, the company took a significant innovative step for being the first in the world in launching POCT Solution for variant B.1.1.7 (VUI 202012/01) and wild-type virus differentiation (https://bit.ly/3qDsmky). These solutions using POCT mechanism to offer additional values to the variant diagnosis using genome sequencing and PCR technologies, being an excellent mean to fight against the global-spread of mutant viruses.
New challenges in E484K and other mutant strains identification
The
'escape mutation' E484K that firstly found in 501Y.V2 (B.1.351)
and 501Y.V3 (B.1.1.28) variant strains in the South African and Brazilian has now spread
to UK, Mayotte, Belgium, France, Switzerland and other countries
according to GISAID.
E484K is named 'an
escape mutation' because it helps the virus bypass the body's immune defences
and weakens the protective effect of some vaccines. In February, South Africa
halt its rollout of the Oxford-AstraZeneca vaccine after a study showed
"disappointing" results against the new variant. A study from Cambridge University has even
confirmed the combination of more transmissible variant B.1.1.7 and E484K
mutation substantially increases the amount of serum antibody needed to prevent
infection of cells.
Moreover, the Manaus city in Brazil that thought to reach herd immunity in the first wave are
now experiencing a second outbreak, and the situation is fuelling by the
COVID-19 variants. However, only 0.03% of cases in Brazil
underwent genomic sequencing for variant identification due to the lacking of
resources.
The fight of COVID-19 is facing new challenges caused by complex variants and mutation. As a mainstream COVID-19 raw material supplier to the global top-profile reagent manufacturers, Fapon Biotech continues to keep a close watch on the ever-changing pandemic situation and introduce products for other mainstream strain identification.
Fapon Biotech COVID-19 Variant E484K POCT Solution
Products and technical services offer to aid the development of rapid, low-cost and large-scale POCT E484K mutant strains detection.
Principle
Used E484K mutant strain recombinant RBD antigen to detect the escape phenomenon of antibodies produced by the non-E484K mutant strain infection to distinguish E484K and non-E484K mutant strain.
- T1 Non-E484K mutant strain recombinant RBD antigen
- T2 E484K mutant strain recombinant RBD antigen
Result Illustration
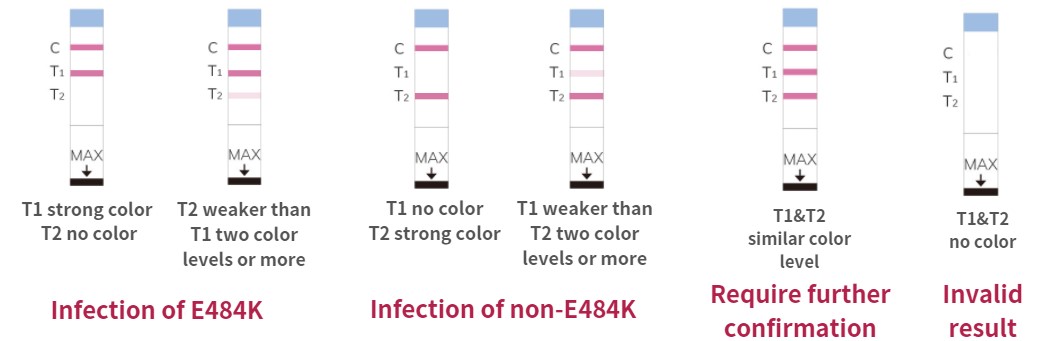
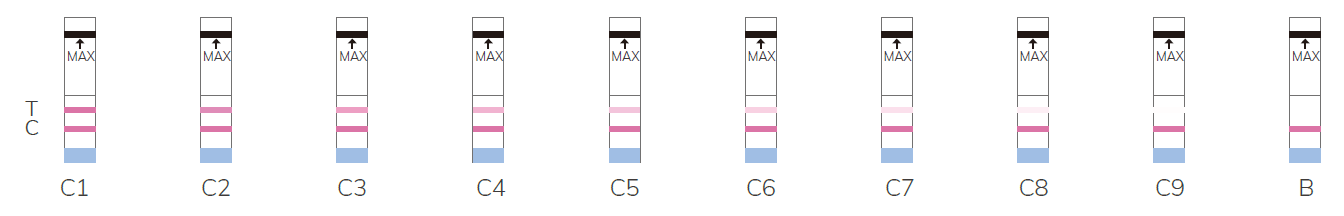
Note: the
higher the number, the lower the activity, B refers to undetectable
Product Information
|
Product Description |
Purity |
Application |
|
Non-E484K mutant strain recombinant RBD antigen |
≥90% |
Coating antigen 1 (T1) |
|
E484K mutant strain recombinant RBD antigen |
Coating antigen 2 (T2) |
|
|
ACE2 antigen |
Conjugate |
Performance
Type of
Verification
Sample
Quantity
Result
Negative
blood sample
Random clinical
sample
198
T1&T2 color level within C4 to C5+
Hypertensive patient sample
72
Positive pneumonia
patient sample
39
COVID-19 patient serum
Non-E484K mutant
strain sample
17
T1 weakens
than T2 two color levels or more
E484K mutant strain
sample
Coming soon