Related Suggestion
Fapon Biotech new reagent raw materials and test components for the latex-enhanced immunoturbidimetric platform is now added to the COVID-19/SARS-CoV-2 Neutralizing Antibody Test Solution. Products for ELISA, CLIA and CMIA, had introduced earlier in supporting customers’ NAb test development and vaccine effectiveness evaluation. With the company mission, ‘Enable Earlier Disease Identification, More Convenient, Accurate and Affordable Diagnosis’, continues to illuminate each action made during the pandemic, Fapon Biotech seeks ongoing innovations to support pandemic diagnosis and control.
Current Challenge in Vaccine Effectiveness Evaluation
The current gold standard method uses the live virus neutralization model for NAb detection in vaccine clinical trials, which requires BSL3 laboratory cultivation and can not be applied as a large-scale conventional testing method. Although a BSL3 laboratory does not require in alternative methods pseudovirus-based and GFP/Luciferase gene reporter system, the detection time that takes 3 to 5 days also sets the limit on their application as large-scale conventional tests.
Fapon Biotech COVID-19 NAb Test Solution
Fapon Biotech COVID-19 NAb Test Solution provides alternative testing methods to gold standard live virus-based PRNT with high correlation in performances, solving the problems of requiring high laboratory safety level, long detection time and quantification. Similar products are launched previously for the application of ELISA, CLIA and CMIA platforms (http://bit.ly/3d0QIBb). New products for Latex-Enhanced Immunotrurbidimetry platform are further introduced to diversify the Fapon Biotech COVID-19 NAb Test Solution, offering more options to develop high-throughput, accurate and convenient NAb commercial test kits.Fapon Biotech COVID-19 NAb Test Solution for Latex-Enhanced Immunoturbidimetry
1. Innovative patented methodology for both neutralizing antibody and total antibody detection
2. Quantitative analysis and dynamic monitoring of vaccine antibody titer and effect evaluation
3. Less detection time, no P3 lab required, break the limitations of testing requirement on environment and personnel
4. Achieve high-throughput detection, meet the needs of different application scenarios
5. High versatility to meet applications in automatic/semi-automatic biochemical instruments, specific protein analyzers, blood-cell counters and other detection platforms
Product Information
Cat. No
Product
Name
Product
Form
Component
Parameter
Applicable
Analyzer
S03001
COVID-19 NAb & Total Ab Test Kit
OEM
R1/R2/R3
15:135:15:50; 700nm
Automatic Biochemical
Instruments
S03001C
Calibrator
C1-C6 (FPZ0570)
Spline
S03001Q
QC
L/H (FPZ0570)
-
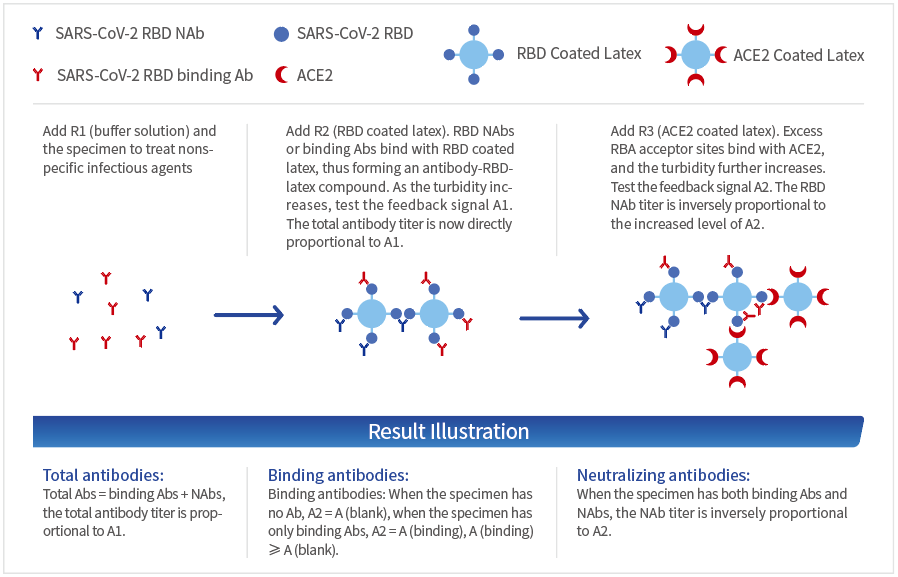
Principle
Performance
Parameters
Analyzer
Sample
Sample size
R1 (buffer)
R2 (RBD latex reagent)
R3 (ACE2 latex reagent)
Wavelength
Testing method
Calibration
Automated Biochemistry Analyzer
Human serum
15μL
135μL
15μL
50μL
700nm
Endpoint
Spline
Specificity & Cut-off Value
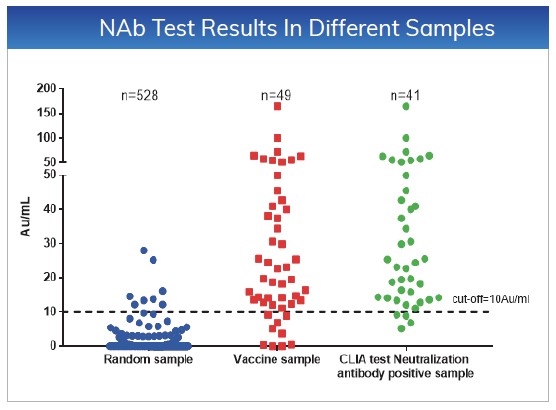
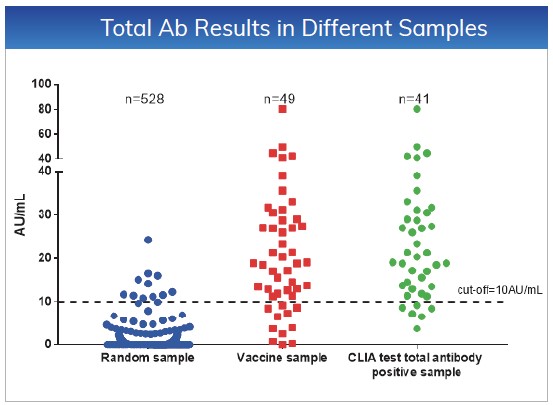
The cut-off values for NAb and Total Ab
are ≤10AU/mL. Tested 528 random samples, the specificity for NAb and Total
Ab is 98.48% and 97.92%
Linearity & Hook Curve
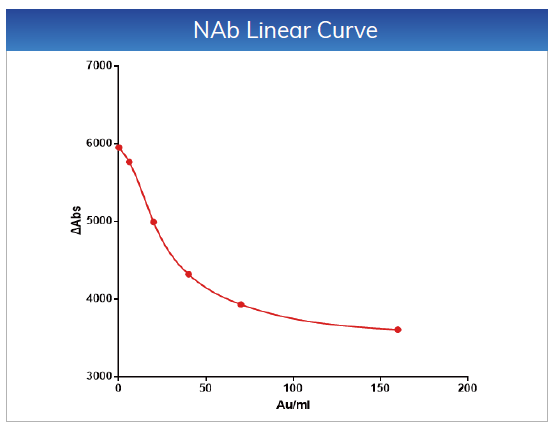
For NAb, the linear range is within 4-160 AU/mL,
the linear correlation coefficient is r > 0.990, the absolute deviation is ≤
±1AU/mL
in the range of 4-10 AU/mL, and the relative deviation is ≤ 10% in the range of
10-160 AU/mL
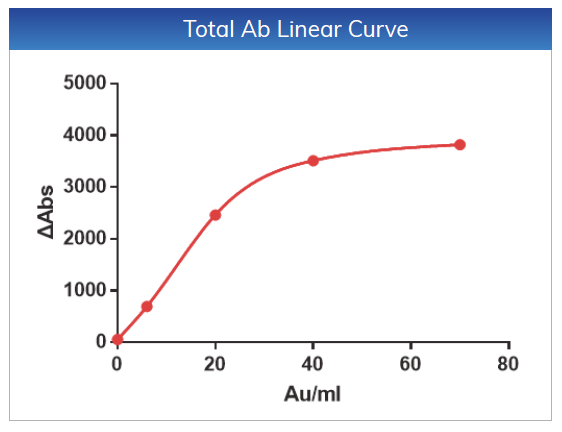
For Total Ab, the linear range is within 2-70
AU/mL, the linear correlation coefficient is r > 0.990, the absolute
deviation is ≤ ±6AU/mL
in the range of 2-6 AU/mL, and the relative deviation is ≤ 10% in the range of
6-70 AU/mL
Clinical Correlation
The Correlation between Fapon NAb immunoturbidimetry and FDA EUA approved competitive enzyme-linked immunoassay is R = 0.73
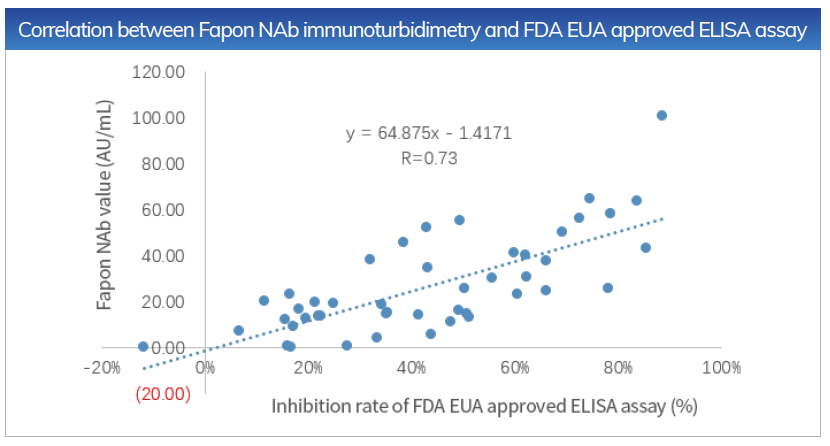
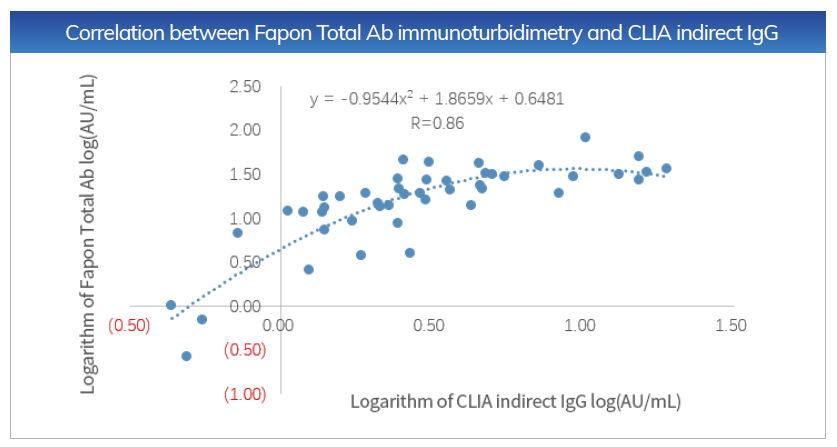
Sensitivity
The sensitivity of NAb and Total Ab is LOQ=4 AU/mL and LOQ=1.076 AU/mL
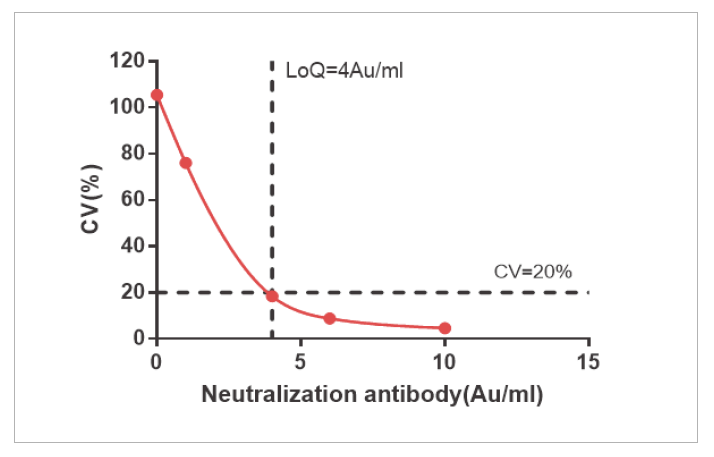
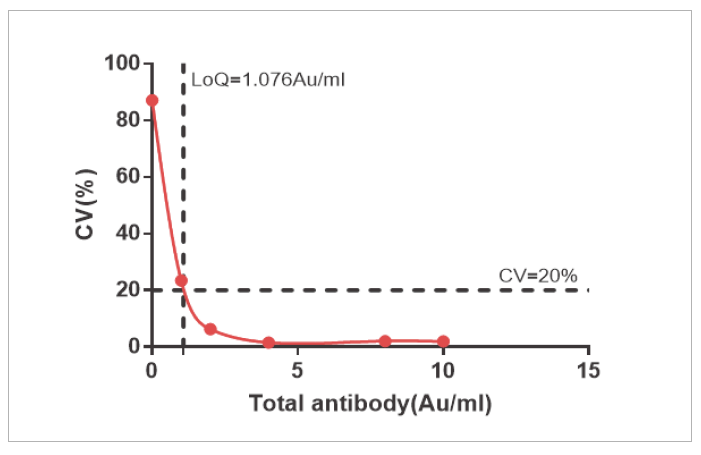
Precision
The precision of
NAb and Total Ab is CV ≤ 6%
Cross-reaction
No cross-reaction was shown in
mycoplasma pneumonia (n=32) and influenza A (n=7) positive samples